
Molécula Del Anión Del Sulfito Sulfitos De Los Sulfitos Que Contienen
Lewis structure of sulfite ion is drawn in this tutorial step by step. Total valence electrons concept is used to draw the lewis structure. Resonance structures of SO 32- are drawn after drawing the lewis structure. Sulfite ion | sulphite ion | SO 32- Sulfite ion is one of the oxyanion of sulfur. Sulfur is at +4 oxidation state in SO 32-.

So3 Lewis Structure With Formal Charges
Chemistry learning made easy.This tutorial will help you deal with the lewis structure and moleculargeometry of sulfite ion (SO3 2-).

Ionic Bonding Elements are the simplest substances There
The hydroxide ion, though, contains an octet of electrons, one more than the neutral molecule. The hydroxide ion must thus carry a single negative charge. In order to draw the Lewis structure for a given ion, we must first determine how many valence electrons are involved. Suppose the structure of H 3 O + is required. The total number of.

So3 схема химической связи 82 фото
A step-by-step explanation of how to draw the SO3 2- Lewis Structure (Sulfite Ion). For the SO3 2- Lewis structure the total number of valence electrons.
[Solved] Draw lewis structure for Sulfite ion SO 3 2 Course Hero
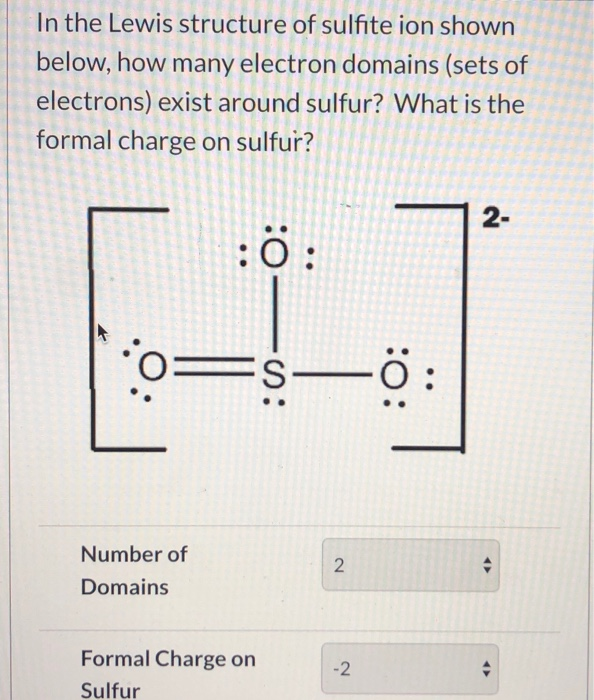
The Lewis structure of sulfite [SO3]2- ion is made up of a sulfur (S) atom and three oxygen (O) atoms. The sulfur (S) is present at the center of the molecular ion while oxygen (O) occupies the terminals, one on each side. There are a total of 4 electron density regions around the central S atom in the Lewis structure of [SO3]2-.

Hướng dẫn vẽ cấu trúc Lewis của so4 2 lewis structure chi tiết và dễ hiểu
Lewis Dot Structure of SO3 2- (Sulfite Ion) kentchemistry.com 25.1K subscribers Subscribe Subscribed 196K views 12 years ago Every Video I quickly take you through how to draw the Lewis.
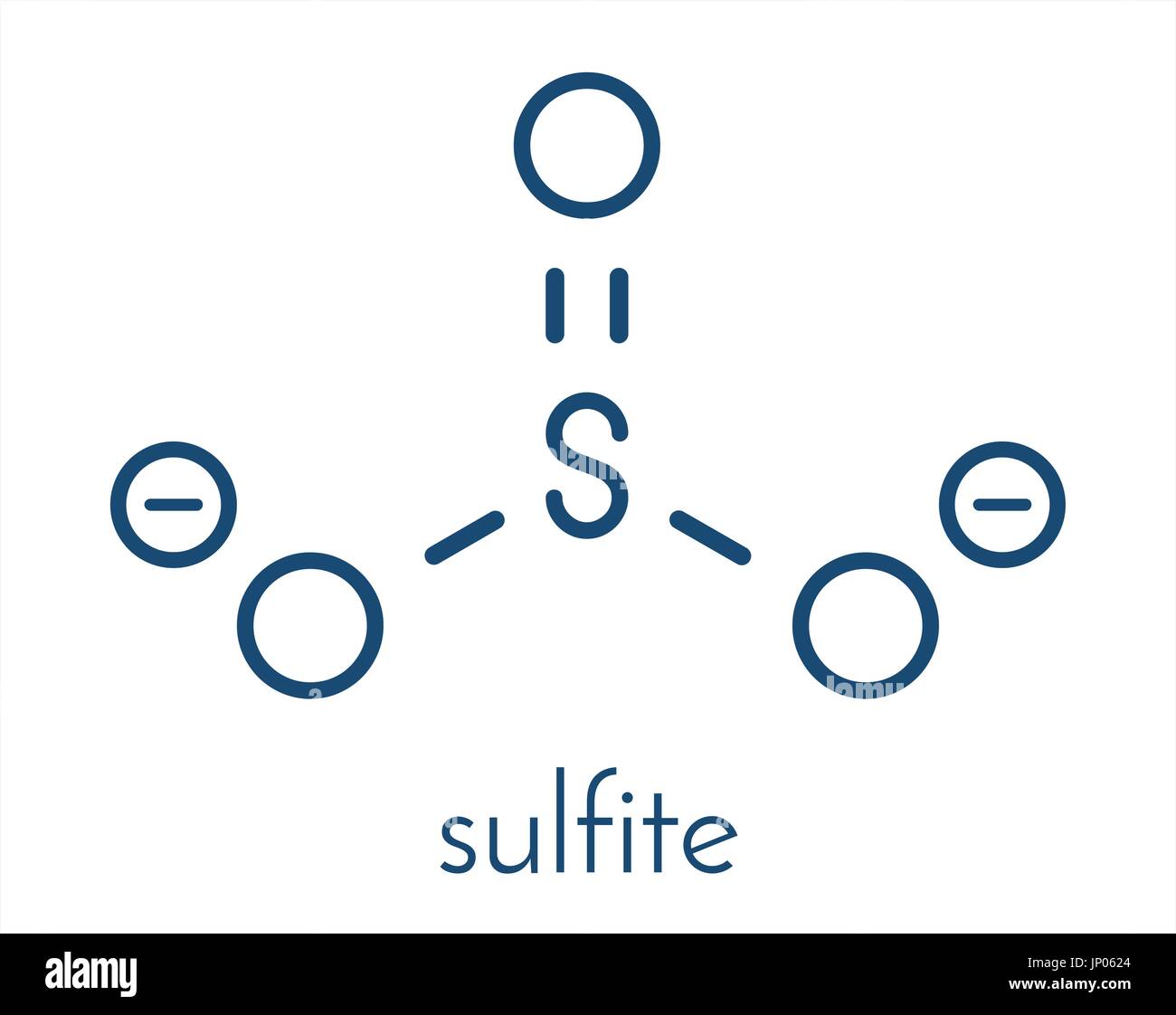
Sulfite anion, chemical structure. Sulfite salts are common food
Video 8.5.2: Please look at Handout 1: Lewis dot structure technique while watching this video. Note steps 1 and 2 are switched but all the rest are the same. Question: What is the formal charge of each atom in the Lewis dot structure of sulfite? Answer The sulfur has a [+1] formal charge as it donates 6 electrons to the structure, but has 5.
[Solved] Draw lewis structure for Sulfite ion SO 3 2 Course Hero
Lewis Dot of the Sulfite Ion SO 32- Back 70 More Lewis Dot Structures S does not follow the octet rule. It will hold more than 8 electrons. Sulfur having valence electrons in the 3rd energy level, will also have access to the 3d sublevel, thus allowing for more than 8 electrons.
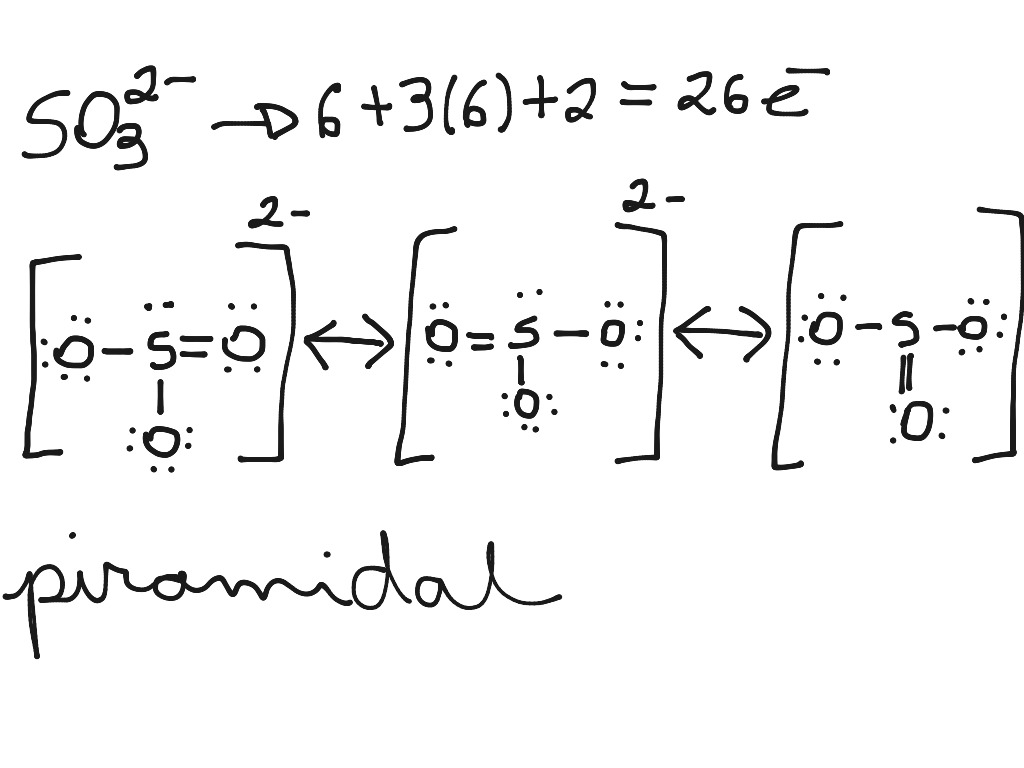
Sulfite ion Science, Chemistry, Chemical Bonds ShowMe
The structure of the sulfite anion Sulfite is a ligand in coordination chemistry. The structure of Co ( ethylenediamine) 2 (SO 3 )N 3. [4] The structure of the sulfite anion can be described with three equivalent resonance structures.
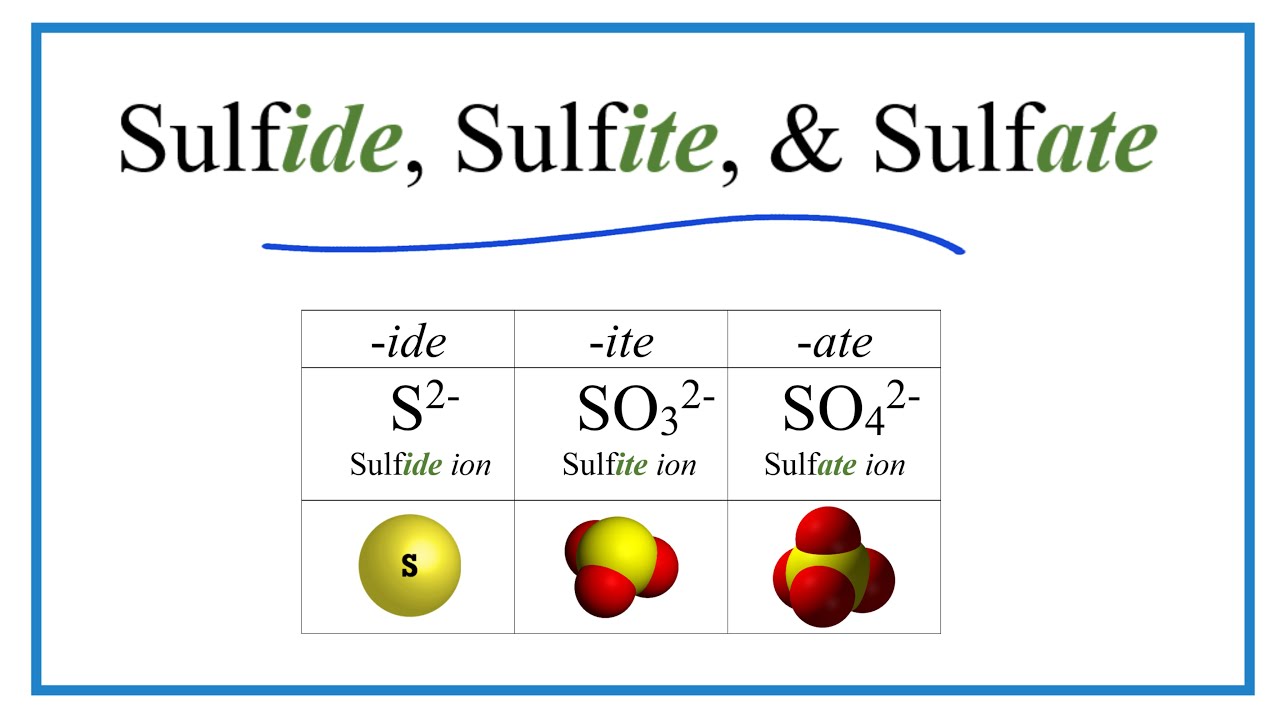
Sulfide, Sulfite, Sulfate Ions (Difference And Formulas) vlr.eng.br
Lewis structure that minimizes formal charges Lewis structure that satisfies the octet rule The sulfur-oxygen bond order is 1.33. The sulfur-oxygen bond order is 1.00. Problem 4. The Lewis structure for the ion OCN- has a single bond between oxygen and carbon and a triple bond between carbon and nitrogen.
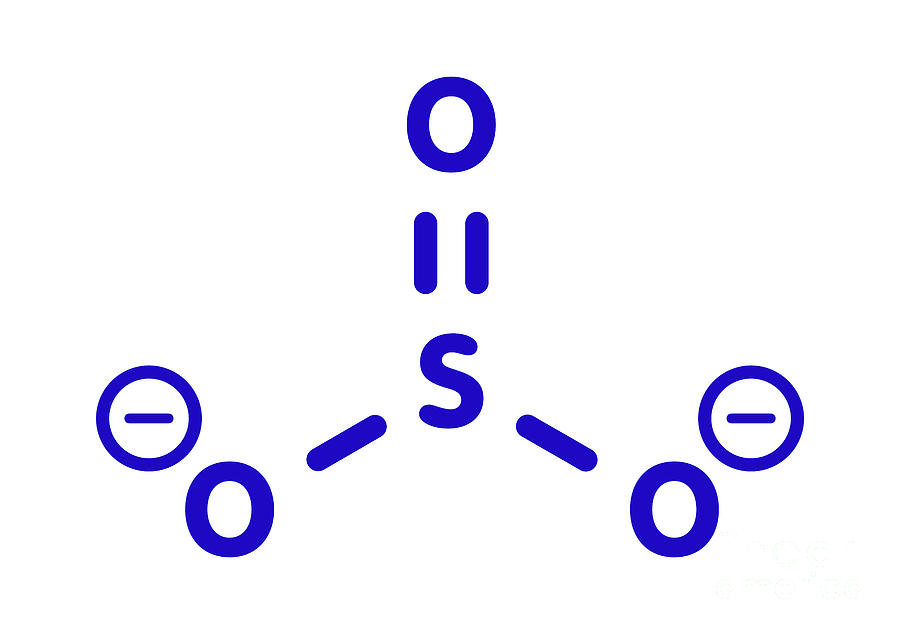
Sulfite Anion Chemical Structure Photograph by Molekuul/science Photo
So this is a possible structure for the sulfite ion, SO3 2-. Since Sulfur is in the third period on the periodic table, it can hold more than eight valence electrons. So we should check our formal charges here to see if this is the best structure. For the Sulfur, on the periodic table, it has 6 valence electrons.

SO3 2 Lewis Structure (Sulfite Ion) YouTube
We are going to determine the Lewis structure for SO3 2- minus ion, also known as sulfide ion. The -2 charge you see is because it accepts two additional electrons, giving the ion a negative charge. Step-by-Step Guide to Drawing the SO3 2- Lewis Structure 1. Count Valence Electrons
Sulfide Ion Lewis Structure My XXX Hot Girl
How to Draw the Lewis Dot Structure for S 2- (Sulfide ion) Wayne Breslyn 724K subscribers Subscribe Subscribed 461 103K views 5 years ago A step-by-step explanation of how to draw the S2-.

FileSulfateion2Ddimensions.png Wikimedia Commons
The sulfite ion comprises one Sulfur Atom and three Oxygen atoms. The ion has a negative.more.more Hello Guys!The sulfite ion comprises one Sulfur Atom and three Oxygen atoms. The.

Sulfur S (Element 16) of Periodic Table Elements FlashCards
Sulfite ion is a weak base, but does undergo some hydrolysis to produce basic solutions. In acidic solution, the equilibria are shifted to form sulfurous acid, resulting in the evolution of SO2 gas. Sulfur dioxide is a colorless gas with a characteristic choking odor. SO2−3 (aq) +H2O(l) ↽−−⇀ HSO−3 (aq) +OH−(aq) SO 3 2 − ( aq.

Lewis Dot Diagram For Sulfur Wiring Diagram
It is useful to see what can be conveyed via 3D models, molecular geometry for instance, and what is conveyed by Lewis structure models, an example being the assignment of electrons around atoms and assignment of electrons for bonding. An important clarification, is that the twirlymols display connections, not bonds, so double and triple bonds.